Table of Contents
Left: RFNA. Right: fuming nitric acid (forget if it was RFNA or WFNA)
Applications
Includes:
- Epoxy decapsulation
- Etch solutions (ex: HNA)
Storage
I've had the best luck storing it in actinic (tinted) glass with a PTFE stopper. Although, I've only used the PTFE stoppers because it came with the bottles. PTFE has a high CTE and a glass stopper would probably work better.
Update: more recently I've been storing bulk WFNA in Nalgene PFA bottles with ETFE lids. I was originally afraid that the WFNA would outgas and with a stopper there is a natural way to release pressure. However, I haven't observed significant outgassing and thus this seems to be a superior way as its:
- Shatter resistant
- Spill resistant
- Seems to offer decent UV protection
Standard glass seems to work decently but I found that it decomposed quicker. If it was stored in a cabinet it should be fine.
Above: RFNA stored in a standard wheaton bottle. I had 70% stored in an identical bottler for over a year and had no problems. Unfortunately, RFNA is much more potent and the fumes ate through in a matter of days. Upon mild pressure the top collapsed altogether.
Suppliers
DudaDiesel sells this in 63% concentration but can sell you higher concentrations if you ask nicely / pay extra.
I do not know of any US suppliers that will sell RFNA to the average joe.
Safety
HNO3 fumes are very strong and should not be inhaled or come in contact with skin. RFNA especially releases NO2 fumes.
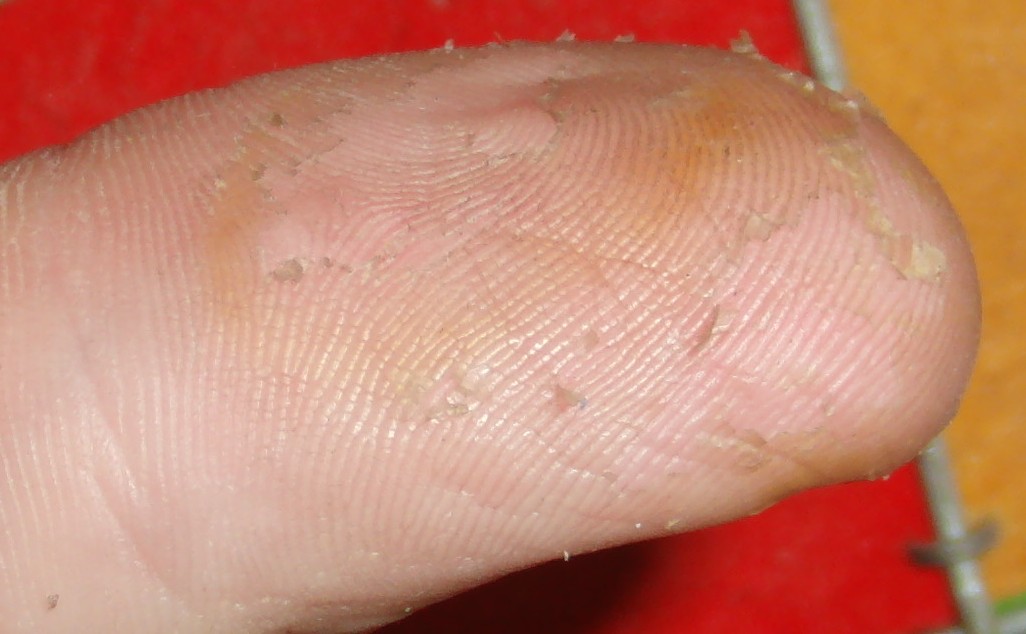
Nitric acid dries out skin and turns it yellow. Takes two or three weeks to heal completely. Example of nearly healed / peeling thumb:
I find that using a 3M full faceplate respirator helps enormously.
Gloves
Both latex and nitrile is eaten by WFNA. The areas of nitrile that aren't eaten completely eaten are weakened and must be replaced. If fuming acid contacts latex/nitrile gloves, try to remove them quickly as it may eat through in a matter of a few seconds. Likely the acid that makes it through won't be too strong but it will discolor skin and dry it out.
Ultimately I use latex gloves because I often use acetone which rapidly eats nitrile gloves. The above thumb picture was caused by acetone weakening a glove and subsequently causing nitric acid exposure.
Compatibility
Always test materials for compatibility before assuming that it will play well with nitric acid, especially the fuming variety. In general, oxidizers, organics, and bases react violently with concentrated nitric acid. Some key materials:
- Alcohol: reacts strongly, even at 70%
- Acetone: less strong reaction than alcohol, but fuming will self heat leading to explosion
- Nitrile: eats through
- Latex: eats through
- PTFE: teflon's nearly invincible so mostly no reaction. However, some reactions self heat enough to cause it to decompose. Particularly can be an issue when decapsulating ICs as it produces HF which is not only toxic but also etches the dies