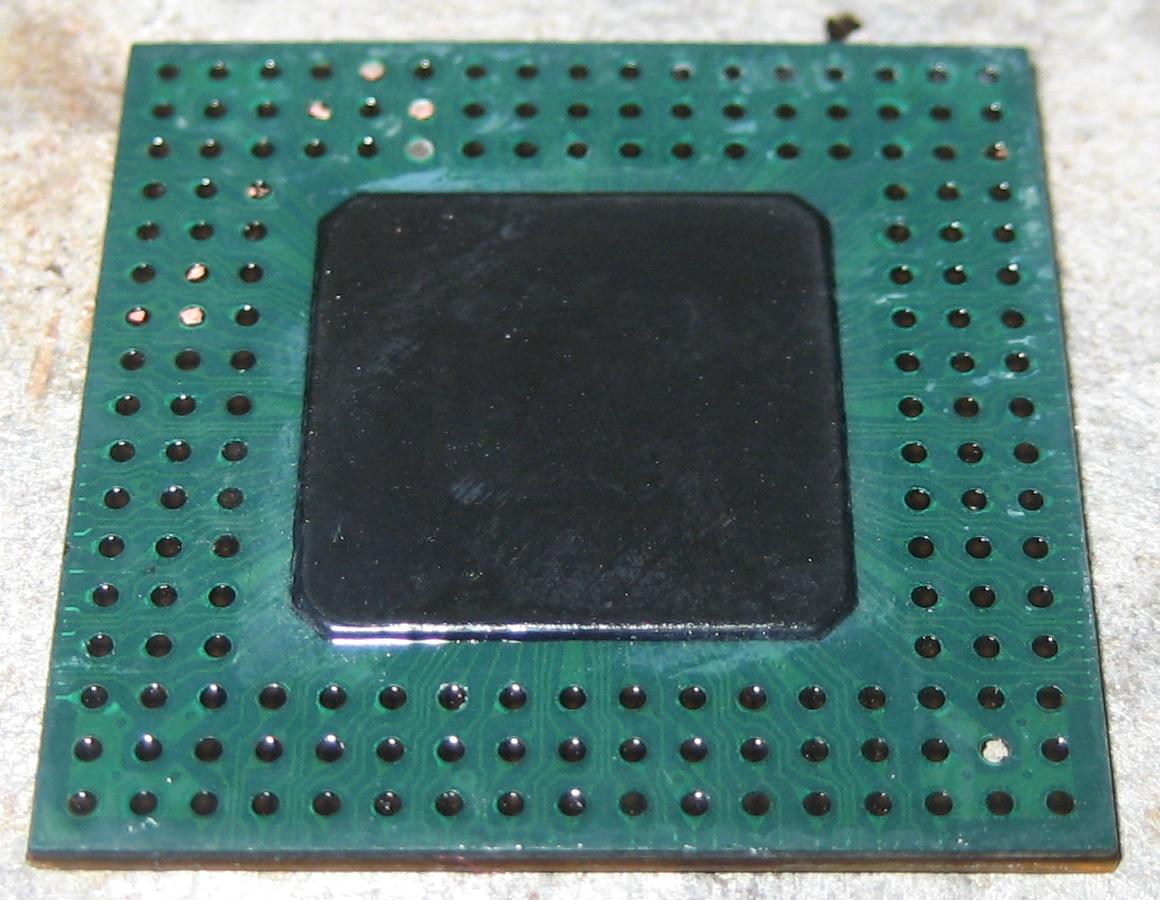
Sample silver plated copper IC:
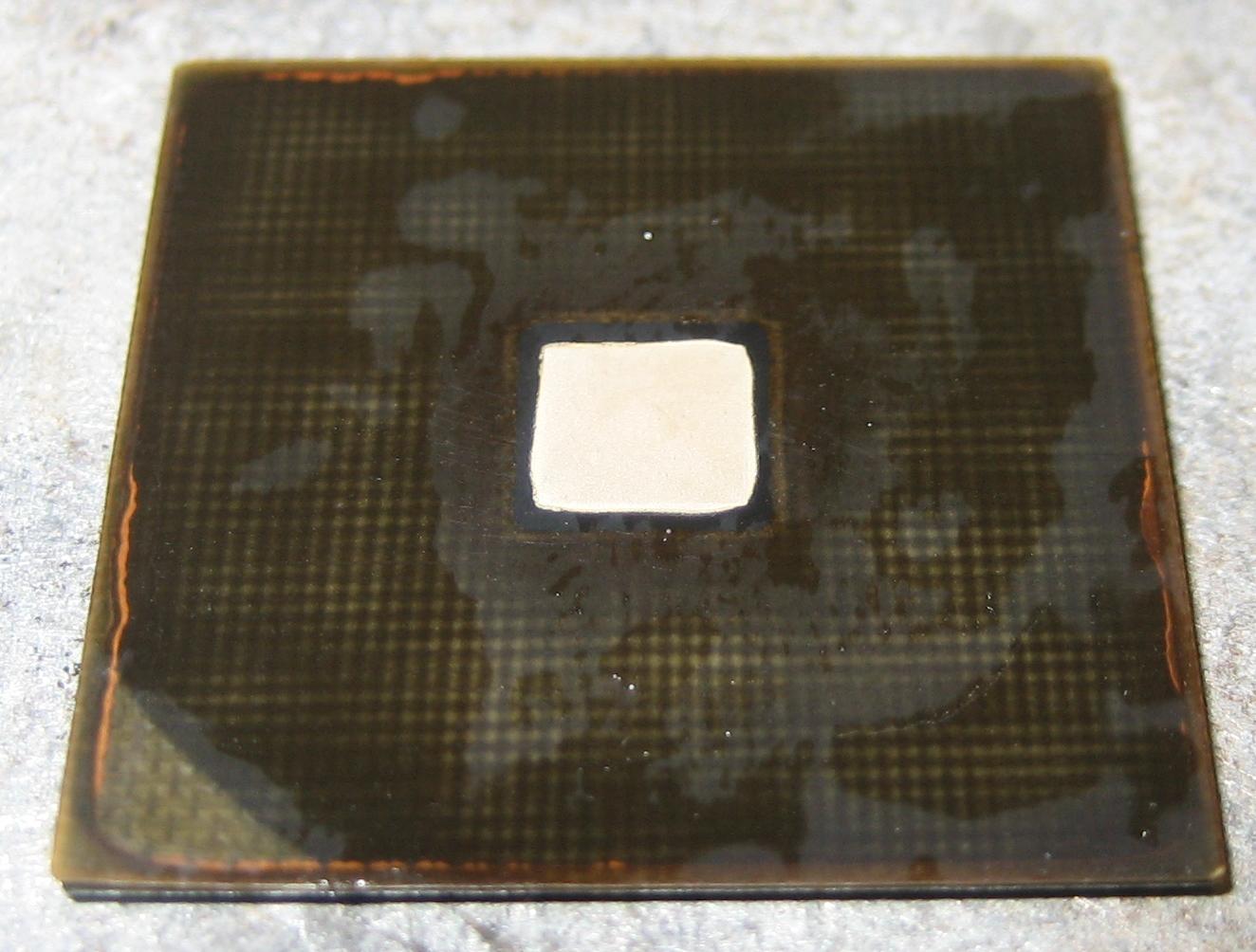
Reverse side looks something like this (picture is missing pins from after etching):
These can be removed using something that eats silver and copper. Nitric acid should work fine, but HCl/H2O2 is more readily available, so it is the preferred method for small labs.
Equipment
- Acid resistant container, eg: beaker
- Stirrer (optional)
- Stir bar (optional)
Chemicals
- H2O2, 3% w/v should be fine
- HCl, any hardware grade
Mix equal parts HCl and H2O2 until the solution covers the IC when its standing on its side. I don't know what the ideal mixture is, but both are cheap so its not really worth the time investigating as this seems to work well enough. Possible literature source could be people who use it for home PCB fabrication. The etch step can do done in two ways:
- Let it sit overnight in solution. Poor H2O2 flow will cause this to take much longer
- Use a stir bar to circulate the solution (recommended)
Following is for second technique. Position the IC so that it is on the side of the beaker. This will allow the solution to flow without the star bar banging against the IC:
Turn on the stir bar at a low speed, thorough stirring is not required. The copper should shimmer as layers are alternatively eaten away and oxidized. Both states can be seen in picture above which was taken during transition. Either periodically check the solution or just wait a few hours and come back. The waste solution should now be much darker:
The IC should look now something like this:
The layers should now be loose and easy to physically pull apart with moderate force. The location in the lower left hand corner looks different because I started to pull it up. Start with the top and bottom layers. The top layer, made of an organic material, should come off fairly easy. The bottom layer, made of FR-4 or similar where the contacts were, should be slightly harder, but not difficult to pull off. You should be left with a middle epoxy coated epoxy layer with a die in the middle. The epoxy bond around the die should be weak and the die can be worked out with minimal force or cut out with an X-Acto knife. After delayering, you should get something like this:
The die should now have only a small layer of epoxy on it and can be dissolved using standard epoxy removal techniques, such as nitric acid.